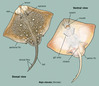
Raja clavataMaiden ray(Also: Rocker; Thorny)
Geographic Range
Raja clavata is wide spread in coastal waters from Iceland to Norway. Its geographic ranges extends into the North Sea, the Mediterranean Sea, the western Black Sea, Madeira Island, the Atlantic coasts of Africa, as well the waters off of South Africa and the south-western Indian Ocean. Adult R. clavata remains inshore during summer and move off shore into deeper waters during winter. (CHEVOLOT, et al., 2006a; CHEVOLOT, et al., 2006b; Ellis, 2005)
- Biogeographic Regions
- palearctic
- ethiopian
- mediterranean sea
Habitat
Raja clavata lives at the bottom of the continental shelf and upper slope of the coastal sea floor. It inhabits a wide range of sea floor habitats including mud, sand, shingle, gravel and rocky areas. Raja clavata has been found in coastal waters as deep as 300 m but is most abundant in waters between 10 and 60 meters. Juveniles are often found further inshore in more shallow waters than their adult counterparts. ("ARKive Images of Life on Earth", 2006; "ICES-Fish Map", December 2005; Ellis, 2005)
- Habitat Regions
- saltwater or marine
- Aquatic Biomes
- coastal
-
- Range depth
- 10 to 300 m
- 32.81 to 984.25 ft
-
- Average depth
- 60 m
- 196.85 ft
Physical Description
Raja clavata shares its general body shape with all rays in that it is a flattened, disc shape with broad pectoral fins connected to the head and body with triangular pelvic fins at the base of the body. Raja clavata has a long, narrow tail that is roughly equivalent to the length of the body. As the common name denotes (thornback ray), the upper portion of the body and tail are covered with thorn-like projections. When R. clavata reaches sexual maturity the bases of the thorns thicken to resemble small button like projections called buckler. The snout and small portions of the body are covered in thorns in sub adults and the underside may be thorny in large females. Adults typically have between 21 and 25 large thorns running from the nape to its first pectoral fin. It is sexually dimorphic, as females have a maximum length of 118 cm and males have a maximum length of 98 cm. The dorsal surface of R. clavata exhibits a wide range of coloration from light brown to grey and is sprinkled with light and dark spots of varying size. Its underside is solid white except for the snout which is grey. The largest specimen ever recorded weighed 18 kg. ("ARKive Images of Life on Earth", 2006; "Raja clavata Linnaeus, 1758 Thornback ray", October 6, 2010; Ellis, 2005)
- Other Physical Features
- ectothermic
- heterothermic
- bilateral symmetry
- Sexual Dimorphism
- female larger
-
- Range mass
- 18 (high) kg
- 39.65 (high) lb
-
- Range length
- 98 to 120 cm
- 38.58 to 47.24 in
Development
Young Raja clavata hatch at 11 to 13 cm in length and 8 to 9 g in weight with a male:female sex ratio of 1:1. Juveniles remain in shallow water. As they increase in size, juveniles grow large button like structures called bucklers. When R. clavata reaches sexual maturity, usually around 8.8 years, it begins seasonally migrating. During summer, adults move in shore during the summer and migrate into deeper waters during the winter. (Ellis, 2005; Nottage and Perkins, 2006)
Reproduction
Little is known of the mating behavior of many skates, including Raja clavata. Some speculate that this is because the animals mate in the cover of night. Studies of sting rays may provide insight into the mating behaviors of elasmobranchs. During mating, females swim in shore and spend the morning either buried in the sand or lay atop each other in large groups. Those females buried are attempting to avoid males that already mated or are not yet ready for reproduction whilst those in the aggregate groups are prepared for mating. Meanwhile, males swim up and down the beach searching for potential mates. In the afternoon, they switch roles and the males bury themselves in the sand while the females become mobile to search for food in the grassy beds. This cycle can continue for multiple weeks. (Smith, 2004)
Thornback rays are oviparous with females migrating further in shore to lay their eggs. Egg-laying season for Raja clavata occurs between March and September. Mature females deposit one egg at a time in sandy or muddy substrate close to shore. Eggs range in length from 5 to 9 cm long and 3.8 to 6.4 cm wide. Mature females can lay between 140 and 160 eggs in a single year. Eggs are rectangular and oblong in shape and are marked by rigid horns on each corner. During development, embryos feed solely on their yolk. Embryonic development typically takes between 4 and 6 months and is largely dependent on ambient water temperature. ("ARKive Images of Life on Earth", 2006; "ICES-Fish Map", December 2005; "Raja clavata Linnaeus, 1758 Thornback ray", October 6, 2010; Ellis, 2005; Holden and Vince, 1973)
- Key Reproductive Features
- iteroparous
- gonochoric/gonochoristic/dioecious (sexes separate)
- sexual
- fertilization
- oviparous
-
- Breeding season
- Thornback rays breed from March to September.
-
- Range number of offspring
- 140 to 160
-
- Range gestation period
- 4 to 6 months
-
- Average age at sexual or reproductive maturity (female)
- 8.8 years
-
- Average age at sexual or reproductive maturity (male)
- 7.1 years
There is no information available regarding parental care in Raja clavata; however, studies on closely related elasmobranches indicate a high level of parental investment. (Ellis, 2005; Payne, et al., 2008)
Lifespan/Longevity
In the wild, Raja clavata lives between 12 and 15 years. There is no information available regarding the average lifespan of captive individuals. ("ARKive Images of Life on Earth", 2006; Holden and Vince, 1973)
-
- Typical lifespan
Status: wild - 12 to 15 years
- Typical lifespan
Behavior
Raja clavata can often be found lying motionless on the bottom of the sea floor, drawing water into its gill slits through a spiracle located behind its eye. It travels in groups of similar sized fish. While traveling, R. clavata use a rajiform swimming method that is common among skates and rays. To achieve directional locomotion, R. clavata simultaneously undulates both pectoral fins in a wave-like manner. ("ICES-Fish Map", December 2005; Ellis, 2005; Hunter, et al., 2005)
- Key Behaviors
- natatorial
- motile
- migratory
- social
Home Range
There is no information available regarding the average home range size of Raja clavata.
Communication and Perception
Like most other elasmobranchs, Raja clavata uses a diverse array of senses that allow it to perceive orientation as well as locate potential predators and prey. In order locate predators and prey, it depends on its acute sense of smell and a lateral line system that allows it to detect changes in pressure in the local environment. The lateral line system is made up of individual mechano-receptors called neuromasts which run the length of the body. These neuromasts are hair filled structures surrounded with a jelly like substance. These are connected to lateral line canals which interact with the environment. Pressure changes in the water displace the hair and fires impulses to the organism’s brain alerting it to the direction and strength of the disturbance. Raja clavata also depends on electrosensory perception organs known as Ampullae of Lorenzini. The ampullae, which consists of a series of gelatin filled pits that create an electrically sensitive network along the ventral surface of the animal, help sense minor electrical pulses created by the muscle contractions of organisms in the local environment. The ampullary organs interact with local electric fields through an opening in the skin on the snout of the animal that leads to a jelly filled canal. The electrical fields produced by animals dissipate rather quickly so R. clavata can only perceive the weak current at short range. Within that short range the thornback ray can accurately determine the location of the prey, even if buried in the sand. Some studies have indicated that R. clavata as well as other sharks and rays can use their Ampullae of Lorenzini to detect the strength and direction of the Earth’s magnetic field as a sort of rudimentary positioning system. Studies have shown that the thornback ray does not rely sight for locating prey. (KALMIJN, 1971; Lowenstein and Roberts, 1950; Lowenstein, et al., 1964; Maruska, 2001; Parker, 1905)
Raja clavata acquires information from its three otilith organs (the utriculus, the sacculus, and the lagena) to maintain spatial equilibrium in its marine habitat. These organs are located in the inner ear vestibular system where the semicircular canals converge next to the cochlea. The three organs respond to tilts in both the longitudinal and transverse axes. The utriculus and sacculus produce similar responses and thus create mutually reinforcing signals. The macula lagena is made up of gravity receptors which provide a marker for R. clavata upright position. The macula utriculus produces an increase in discharge to signal side-up and nose-up displacements while the macula sacculus signals side-up and nose-down displacements. All three maculae act as an "out-of-position receptor" when the individual is subjected to a constant speed spatial deviation. (KALMIJN, 1971; Lowenstein and Roberts, 1950; Lowenstein, et al., 1964; Maruska, 2001; Parker, 1905)
Thornback rays have a very acute sense of smell that they use for locating prey. They have two openings called nares that are located ventrally on the organism’s snout. Water enters the nares and passes through a structure known as the olfactory sac. The olfactory sac is lined with a series of folded tissue called the olfactory lamellae which provide increased surface area for molecule-receptor interactions. Molecules dissolved in the water bind to neuroreceptors in the olfactory lamellae to provide chemosensory information to the brain. (KALMIJN, 1971; Lowenstein and Roberts, 1950; Lowenstein, et al., 1964; Maruska, 2001; Parker, 1905)
Food Habits
Raja clavata is carnivorous. Juveniles feed predominantly on bottom dwelling aquatic crustaceans such as amphipods, mysids and crangonid shrimps. Larger adults feed on larger crustaceans such as swimming crabs and fish, such as sandeels, small gadoids and dragonets. (Ellis, 2005; "ICES-Fish Map", December 2005; Ellis, 2005)
- Animal Foods
- fish
- aquatic crustaceans
Predation
Raja clavata eggs are preyed upon by some fish and mollusks, such as necklace shells. Juveniles are commonly preyed upon by larger fish, specifically common skates and adult R. clavata. The coloration of R. clavata likely helps reduce risk of predation. ("ICES-Fish Map", December 2005)
- Anti-predator Adaptations
- cryptic
-
- Known Predators
-
- necklace shells, Polinices
- common skate, Dipturus batis
- thornback rays, Raja clavata
Ecosystem Roles
Raja clavata feeds on a variety of benthic animals. Juveniles feed on small crustaceans such as amphipods, mysids and crangonid shrimps, and adults feed on fish and larger crustaceans such as swimming crabs and fish such as sandeels, small gadoids and dragonets. This species is also known to feed on conspecifics as well. The eggs of R. clavata act as prey for some fish and mollusks such as necklace shells. Juveniles are preyed upon by common skate and adult R. clavata. Due to its high placement in the costal sea floor food web, R. clavata has a significant effect on trophic levels of the benthos. Parasites of this species have not been documented. (Ellis, 2005)
Economic Importance for Humans: Positive
Raja clavata is more highly valued for its meat than other skates and rays. Thus, R. clavata plays an important economic role in fisheries, particularly those off the coast of Portugal. ("Thornback", 1890; Stevens, et al., 2000)
- Positive Impacts
- food
Economic Importance for Humans: Negative
There are no known adverse effects of Raja clavata on humans. (Ellis, 2005)
Conservation Status
Raja clavata is classified as near threatened on to the ICUN's Red List of Threatened Species. Declines in R. clavata landings are thought to be due to over exploitation and poor species identification. It is caught primarily as by-catch in both otter and beam trawl fisheries, and is targeted with gillnets, long-lines, and recreational anglers. Landings of rays and skates are often lumped together under one generic category (i.e., Raja spp.), but market samplings suggest that R. clavata is the most dominant species landed in the North Sea and Skagerrak. Over exploitation in this species is especially relevant as it has slow growth, long life span, and long period before individuals become reproductively mature make. Research suggests that R. clavata may be extinct from the North Sea due to over fishing. In an effort to help R. clavata recover (as well as other batids), total allowable catch (TAC) for all skates and rays was reduced by 47% from 1999 to 2005. Some fisheries in the United Kingdom have also implemented a minimum landing size. Unfortunately, localized management efforts are not expected to have an impact on regional population conservation. ("ARKive Images of Life on Earth", 2006; "ICES-Fish Map", December 2005; Ellis, 2005; Stevens, et al., 2000)
-
- IUCN Red List
-
Near Threatened
More information
-
- IUCN Red List
-
Near Threatened
More information
-
- US Federal List
- No special status
-
- CITES
- No special status
-
- State of Michigan List
- No special status
Contributors
Jason Dooney (author), The College of New Jersey, Matthew Wund (editor), The College of New Jersey, John Berini (editor), Animal Diversity Web Staff.
Glossary
- Ethiopian
-
living in sub-Saharan Africa (south of 30 degrees north) and Madagascar.

- Palearctic
-
living in the northern part of the Old World. In otherwords, Europe and Asia and northern Africa.

- acoustic
-
uses sound to communicate
- bilateral symmetry
-
having body symmetry such that the animal can be divided in one plane into two mirror-image halves. Animals with bilateral symmetry have dorsal and ventral sides, as well as anterior and posterior ends. Synapomorphy of the Bilateria.
- carnivore
-
an animal that mainly eats meat
- chemical
-
uses smells or other chemicals to communicate
- coastal
-
the nearshore aquatic habitats near a coast, or shoreline.
- cryptic
-
having markings, coloration, shapes, or other features that cause an animal to be camouflaged in its natural environment; being difficult to see or otherwise detect.
- ectothermic
-
animals which must use heat acquired from the environment and behavioral adaptations to regulate body temperature
- electric
-
uses electric signals to communicate
- fertilization
-
union of egg and spermatozoan
- food
-
A substance that provides both nutrients and energy to a living thing.
- heterothermic
-
having a body temperature that fluctuates with that of the immediate environment; having no mechanism or a poorly developed mechanism for regulating internal body temperature.
- internal fertilization
-
fertilization takes place within the female's body
- iteroparous
-
offspring are produced in more than one group (litters, clutches, etc.) and across multiple seasons (or other periods hospitable to reproduction). Iteroparous animals must, by definition, survive over multiple seasons (or periodic condition changes).
- magnetic
-
(as perception channel keyword). This animal has a special ability to detect the Earth's magnetic fields.
- migratory
-
makes seasonal movements between breeding and wintering grounds
- motile
-
having the capacity to move from one place to another.
- natatorial
-
specialized for swimming
- native range
-
the area in which the animal is naturally found, the region in which it is endemic.
- oviparous
-
reproduction in which eggs are released by the female; development of offspring occurs outside the mother's body.
- piscivore
-
an animal that mainly eats fish
- saltwater or marine
-
mainly lives in oceans, seas, or other bodies of salt water.
- sexual
-
reproduction that includes combining the genetic contribution of two individuals, a male and a female
- social
-
associates with others of its species; forms social groups.
- vibrations
-
movements of a hard surface that are produced by animals as signals to others
- visual
-
uses sight to communicate
References
2006. "ARKive Images of Life on Earth" (On-line). Thornback Skate (Raja Clavata). Accessed March 16, 2011 at http://www.arkive.org/thornback-skate/raja-clavata/#text=Facts.
December 2005. "ICES-Fish Map" (On-line pdf). Thornback Ray. Accessed March 16, 2011 at http://www.ices.dk/marineworld/fishmap/ices/pdf/thornback.pdf.
October 6, 2010. "Raja clavata Linnaeus, 1758 Thornback ray" (On-line). Fishbase. Accessed March 16, 2011 at http://www.fishbase.org/Summary/SpeciesSummary.php?ID=2059&AT=Thornback+Ray.
Belford-Clark Company. 1890. Thornback. Pp. 5810 in Americanized Encyclopedia Britannica, Vol. 9. Examiner. Accessed March 24, 2011 at http://books.google.com/books?id=Dj0hAQAAIAAJ&pg=PA5810&dq=encyclopedia+raja+clavata&hl=en&ei=B9KKTd6JA6Sx0QHv3Z2CDg&sa=X&oi=book_result&ct=result&resnum=4&ved=0CEIQ6AEwAw#v=onepage&q=Raja%20clavata&f=false.
CHEVOLOT, M., J. ELLIS, G. HOARARU, R. ADRIAAN, W. STAM, J. OLSEN. 2006. Population structure of the thornback ray (Raja clavata L.) in British waters. Journal of Sea Research, 56/4: 305-316. Accessed February 23, 2011 at http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VHH-4K6KB12-3&_user=1086025&_coverDate=11%2F30%2F2006&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1654040519&_rerunOrigin=scholar.google&_acct=C000051441&_version=1&_urlVersion=0&_userid=1086025&md5=3bd19aaa05e9f97b8b75e1cf5f00891c&searchtype=a.
CHEVOLOT, M., G. HOARAU, A. RIJNSDORP, W. STAM, J. OLSEN. 2006. Phylogeography and population structure of thornback rays (Raja clavata L., Rajidae). Molecular Ecology, 15/12: 3693-3705. Accessed February 23, 2011 at http://onlinelibrary.wiley.com/doi/10.1111/j.1365-294X.2006.03043.x/full.
Ellis, J. 2005. "The IUCN Redlist of Threatened Species" (On-line). Accessed February 23, 2011 at http://www.iucnredlist.org/apps/redlist/details/39399/0.
Holden, M., M. Vince. 1973. Age validation studies on the centra of Raja clavata using tetracycline. ICES Journal of Marine Science, 35/1: 13-17. Accessed February 23, 2011 at http://icesjms.oxfordjournals.org/content/35/1/13.abstract.
Holden, M. 1975. The fecundity of Raja clavata in British waters. ICES Journal of Marine Science, 36/2: 110-118. Accessed February 23, 2011 at http://icesjms.oxfordjournals.org/content/36/2/110.abstract.
Hunter, E., A. Buckley, C. Stewart, J. Melcaffe. 2005. Migratory behaviour of the thornback ray, Raja clavata, in the southern North Sea. Journal of the Marine Biological Association of the UK, 85: 1095-1105. Accessed February 23, 2011 at http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=341571.
KALMIJN, A. 1971. THE ELECTRIC SENSE OF SHARKS AND RAYS. Journal of Exploratory Biology, 55: 371-383. Accessed March 24, 2011 at http://jeb.biologists.org/cgi/reprint/55/2/371.
Lowenstein, O. 1959. A Functional Interpretation of the Electron-Microscopic Structure of the Sensory Hairs in the Cristæ of the Elasmobranch Raja clavata in Terms of Directional Sensitivity. Nature, 184/4701: 1807-1808. Accessed February 23, 2011 at http://adsabs.harvard.edu/abs/1959Natur.184.1807L.
Lowenstein, O., M. Osborne, J. Wersall. 1964. Structure and Innervation of the Sensory Epithelia of the Labyrinth in the Thornback Ray (Raja clavata). Biological Sciences, 160/978: 1-12. Accessed February 23, 2011 at http://www.jstor.org/stable/75390.
Lowenstein, O., T. Roberts. 1950. THE EQUILIBRIUM FUNCTION OF THE OTOLITH ORGANS OF THE THORNBACK RAY (RA7A CLAVATA). Journal of Physiology, 110: 392-415. Accessed March 24, 2011 at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1392752/pdf/jphysiol01470-0148.pdf?tool=pmcentrez.
Maruska, K. 2001. Morphology of the Mechanosensory Lateral Line System in Elasmobranch Fishes: Ecological and Behavioral Considerations. Environmental Biology of Fishes, 60/1-3: 47-75. Accessed April 14, 2011 at http://web.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=87f34ad6-28db-4dd6-8ebc-81b728d02d97%40sessionmgr104&vid=2&hid=113.
Nottage, A., E. Perkins. 2006. Growth and maturation of roker Raja clavata L. in the Solway Firth. Journal of Fish Biology, 23/1: 43-48. Accessed February 23, 2011 at http://onlinelibrary.wiley.com/doi/10.1111/j.1095-8649.1983.tb02880.x/abstract.
Parker, G. 1905. The function of the lateral line organs in Fishes. Bulletin of the Bureau of Fisheries, 24: 185-204. Accessed March 24, 2011 at http://books.google.com/books?id=prIgAAAAMAAJ&printsec=frontcover&source=gbs_ge_summary_r&cad=0#v=onepage&q=Raja%20clavata%20lateral%20line%20system&f=false.
Payne, A., J. Cotter, A. Cotter, T. Potter. 2008. Advances in fisheries science: 50 years on from Beverton and Holt. John Wiley and Sons. Accessed March 24, 2011 at http://books.google.com/books?id=u20mdaUF3zMC&dq=parental+investment+thornback+ray&lr=&source=gbs_navlinks_s.
Rousset, J. 1990. Population structure of thornback rays Raja clavata and their movements in the Bay of Douarnenez. Journal of the Marine Biological Association of the United Kingdom, 70: 261-268. Accessed February 23, 2011 at http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=4368296.
Smith, M. 2004. The elasmobranch husbandry manual: captive care of sharks, rays, and their relatives. the University of California: Ohio Biological Survey. Accessed March 24, 2011 at http://books.google.com/books?id=TpoXAQAAIAAJ&q=mating+systems+skates&dq=mating+systems+skates&hl=en&ei=ecKLTbvIIM2Q0QGilrSgCw&sa=X&oi=book_result&ct=result&resnum=4&ved=0CDwQ6AEwAw.
Stevens, J., R. Bonfil, N. Dulvy, P. Walker. 2000. The effects of fishing on sharks, rays, and chimaeras. ICES Journal of Marine Science, 57/3: 476-494. Accessed March 24, 2011 at http://icesjms.oxfordjournals.org/content/57/3/476.full.pdf+html.
Whittamore, J., I. McCarthy. 2005. The population biology of the thornback ray, Raja clavata in Caernarfon Bay, north Wales. Journal of the Marine Biological Association of the UK, 85: 1089-1094. Accessed February 23, 2011 at http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=341569.
Wilhelmus, J., A. Smeets. 2004. The secondary olfactory connections in two chondrichthians, the shark Scyliorhinus canicula and the ray Raja clavata. The Journal of Comparative Neurology, 218/3: 334-344. Accessed February 23, 2011 at http://onlinelibrary.wiley.com/doi/10.1002/cne.902180309/abstract.